Ying Jiang's group discovered an unconventional ice structure, which completely breaks the ice rules.
Water-solid interactions are of broad importance both in nature and technology. The hexagonal bilayer model based on the Bernal-Fowler-Pauling ice rules has been widely adopted to describe water structuring at interfaces. However, the validity of the hexagonal bilayer model on interfacial water is being challenged due to the subtle balance between the water-water and water-substrate interactions. Recently, the research team led by Prof. Ying Jiang of International Center of Quantum Materials (ICQM) of Peking University, in collaboration with Prof. Enge Wang and Prof. Xinzheng Li from the same center, discovered a highly defective tetramer-based ice structure on NaCl(001) surface, which goes well beyond the simple hexagonal bilayer model predicted by the ice rules. This work was published online in Nature Communications on May 30th, 2014 [Nat. Commun. DOI: 10.1038/ncomms5056].
During the past four years, Ying Jiang’s group has been working on the development of ultrahigh-resolution scanning tunneling microscope (STM) for single-molecule experiments. Recently, they made a breakthrough in achieving submolecular-resolution imaging of individual water molecules adsorbed on a Au-supported NaCl(001) film [Nat. Mater. 13, 184 (2014)]. Such a technique opens up the possibility of determining the detailed topology of H-bonded networks at water/solid interfaces with atomic precision.
As an important application of this technique, Ying Jiang and his collaborators stepped further to study the ice overlayer grown on the NaCl(001) film and discovered an unconventional bilayer ice structure built from cyclic water tetramers at 77 K. The water tetramers within the lower part of the bilayer act as the basic building blocks, which are interconnected via a novel bridging mechanism to form a regular array of Bjerrum D-type defects located in the upper layer. Ab initio theoretical calculations based on density functional theory rationalize the stabilization of such an unconventional bilayer ice and reveal a striking proton-disordered ice structure. Notably, the formation of the periodic Bjerrum defects with unusually high density is strongly against the Bernal-Fowler-Pauling ice rules and may play a crucial role in catalyzing heterogeneous chemical reactions on water-coated salt surfaces as well as in influencing various phenomena such as heterogeneous ice nucleation, salt dissolution and caking.
This work received supports from Ministry of Science and Technology of China, National Natural Science Foundation of China, Ministry of Education of China, and National Program for Support of Eminent Professionals.
Paper link: http://www.nature.com/ncomms/2014/140530/ncomms5056/full/ncomms5056.html
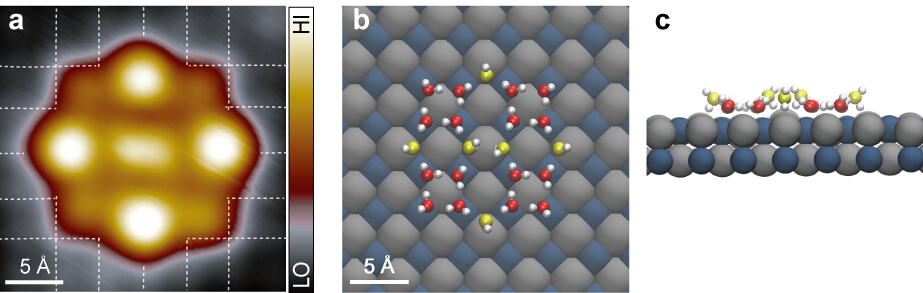
(a) High-resolution STM image of a 2D ice nanocluster consisting of four tetramers and six bridging water molecules. Square lattices of the NaCl(001) surface arising from Cl are depicted by white grids. (b) and (c) Top and side views, respectively, of the calculated adsorption configurations of the ice nanocluster. H, Cl and Na are denoted by white, grey and dark-cyan spheres, respectively. For clarity, the O atoms of water molecules in lower and upper layers are represented by red and yellow spheres, respectively.